|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Chaperones
As you can see when looking through the many structures in the PDB, most active proteins have a stable, globular structure. However, proteins are built as formless chains, pieced together one amino acid at a time by ribosomes. Most protein chains then fold spontaneously into their final structure, driven by the need to shelter their carbon-rich portions from the surrounding water. But some--large proteins or proteins with several domains--need some assistance. As they fold into a compact shape, they might get stuck somewhere along the way.
The Dangers of Misfolding
This is not a trivial problem. Cells cannot merely wait for proteins to fold properly. Misfolded proteins often have carbon-rich amino acids on their surfaces, instead of tucked safely inside. These carbon-rich patches associate strongly with similar patches on other proteins, forming large aggregates. Random aggregates are death to cells: diseases such as sickle cell anemia, mad cow disease, and Alzheimer's disease are caused by unnatural aggregation of proteins into cell-clogging fibrils.Guides Along the Folding Pathway
Chaperones are proteins that guide proteins along the proper pathways for folding. They protect proteins when they are in the process of folding, shielding them from other proteins that might bind and hinder the process. Many chaperone proteins are termed "heat shock" proteins (with names like HSP-60) because they are made in large amounts when cells are exposed to heat. Heat, in general, destabilizes proteins and makes misfolding more common. So when it gets really hot, cells need some extra help with their proteins.HSP-60 Chaperones
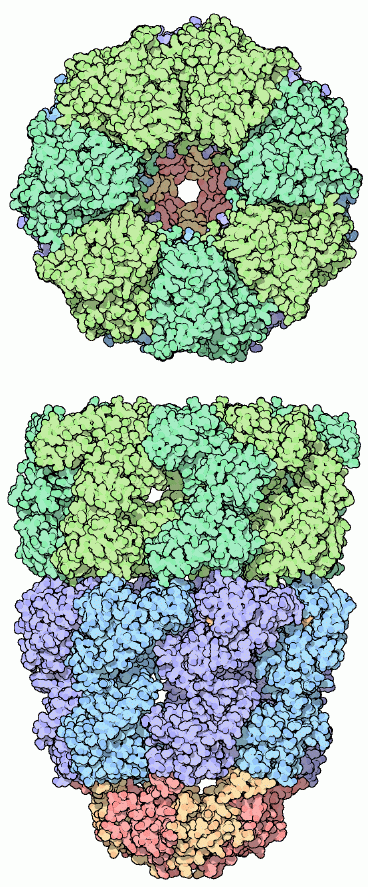
One impressive type of chaperone forms an enclosed environment for folding proteins which totally protects them as they fold. The GroEL-GroES complex of the bacterium E. coli is shown here, from PDB entry 1aon. It is composed of two stacked rings of GroEL proteins, colored blue and green here, and a cap on one side composed of GroES, colored red and yellow at the bottom. As seen in the top view, seven GroEL proteins form a ring with a protein-sized cavity inside. Unfolded proteins enter this cavity and fold up inside.Next: HSP-70 and Prefoldin
Last changed by: A.Honegger,