|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Hemoglobin
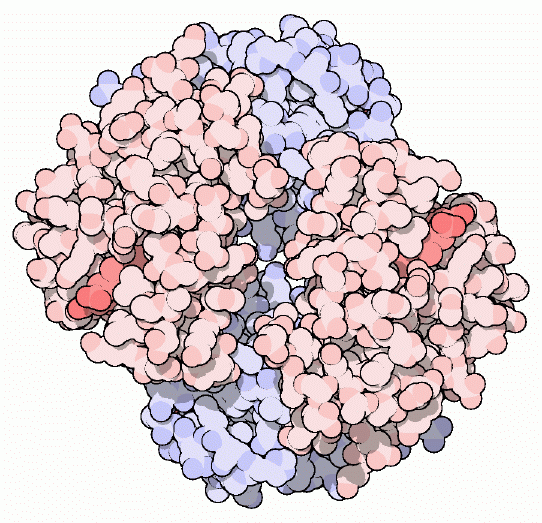
Red Blood, Blue Blood
Ever wondered why blood vessels appear blue? Oxygenated blood is bright red: when you are cut, the blood you see is brilliant red oxygenated blood. Deoxygenated blood is deep purple: when you donate blood or give a blood sample at the doctor's office, it is drawn into a storage tube away from oxygen, so you can see this dark purple color. However, deep purple deoxygenated blood appears blue as it flows through our veins, especially in people with fair skin. This is due to the way that different colors of light travel through skin: blue light is reflected in the surface layers of the skin, whereas red light penetrates more deeply. The dark blood in the vein absorbs most of this red light (as well as any blue light that makes it in that far), so what we see is the blue light that is reflected at the skin's surface. Some organisms like snails and crabs, on the other hand, use copper to transport oxygen, so they truly have blue blood.Hemoglobin is the protein that makes blood red. It is composed of four protein chains, two alpha chains and two beta chains, each with a ring-like heme group containing an iron atom. Oxygen binds reversibly to these iron atoms and is transported through blood. Each of the protein chains is similar in structure to myoglobin (presented in the January 2000 Molecule of the Month), the protein used to store oxygen in muscles and other tissues. However, the four chains of hemoglobin give it some extra advantages, as described on the next page.
Use and Abuse of Hemoglobin
Aside from oxygen transport, hemoglobin can bind and transport other molecules like nitric oxide and carbon monoxide. Nitric oxide affects the walls of blood vessels, causing them to relax. This in turn reduces the blood pressure. Recent studies have shown that nitric oxide can bind to specific cysteine residues in hemoglobin and also to the irons in the heme groups, as shown in PDB entry 1buw. Thus, hemoglobin contributes to the regulation of blood pressure by distributing nitric oxide through blood. Carbon monoxide, on the other hand, is a toxic gas. It readily replaces oxygen at the heme groups, as seen in PDB entry 2hco and many others, forming stable complexes that are difficult to remove. This abuse of the heme groups blocks normal oxygen binding and transport, suffocating the surrounding cells.Artificial Blood
Blood transfusions have saved countless lives. However, the need for matching blood type, the short life of stored blood, and the possibility of contamination are still major concerns. An understanding of how hemoglobin works, based on decades of biochemical study and many crystallographic structures, has prompted a search for blood substitutes and artificial blood. The most obvious approach is to use a solution of pure hemoglobin to replace lost blood. The main challenge is keeping the four protein chains of hemoglobin together. In the absence of the protective casing of the red blood cell, the four chains rapidly fall apart. To avoid this problem, novel hemoglobin molecules have been designed where two of the four chains are physically linked together, as shown in PDB entry 1c7d. In that structure, two additional glycine residues form a link between two of the chains, preventing their separation in solution.Hemoglobin Cousins
Looking through the PDB, you will find many different hemoglobin molecules. You can find Max Perutz's groundbreaking structure of horse hemoglobin in entry 2dhb, shown in the picture here. There are structures of human hemoglobins, both adult and fetal. You can also find unusual hemoglobins like leghemoglobin, which is found in legumes. It is thought to protect the oxygen-sensitive bacteria that fix nitrogen in leguminous plant roots. In the past few years some hemoglobin cousins called the "truncated hemoglobins" have been identified, such as the hemoglobin in PDB entry 1idr, which have several portions of the classic structure edited out. The only feature that is absolutely conserved in this subfamily of proteins is the histidine amino acid that binds to the heme iron.Last changed by: A.Honegger,