|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Hemoglobin
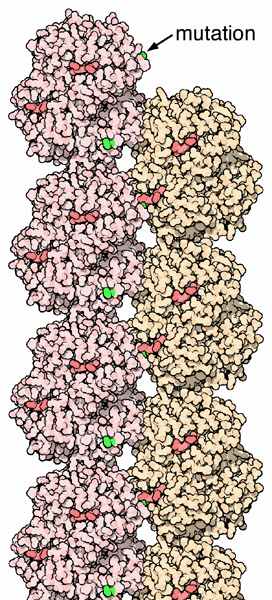
Troubled Hemoglobins
The genes for the protein chains of hemoglobin show small differences within different human populations, so the amino acid sequence of hemoglobin is slightly different from person to person. In most cases the changes do not affect protein function and are often not even noticed. However, in some cases these different amino acids lead to major structural changes. One such example is that of the sickle cell hemoglobin, where glutamate 6 in the beta chain is mutated to valine. This change allows the deoxygenated form of the hemoglobin to stick to each other, as seen in PDB entry 2hbs, producing stiff fibers of hemoglobin inside red blood cells. This in turn deforms the red blood cell, which is normally a smooth disk shape, into a C or sickle shape. The distorted cells are fragile and often rupture, leading to loss of hemoglobin. This may seem like a uniformly terrible thing, but in one circumstance, it is actually an advantage. The parasites that cause the tropical disease malaria, which spend part of their life cycle inside red blood cells, cannot live in the fiber-filled sickle cells. Thus people with sickle cell hemoglobin are somewhat resistant to malaria.Other circumstances leading to troubled hemoglobins arise from a mismatch in the production of the alpha and beta proteins. The structure requires equal production of both proteins. If one of these proteins is missing, it leads to conditions called Thalassemia.
Next: Exploring the Structure
Previous: Cooperation Makes It Easier
Last changed by: A.Honegger,