|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Serpins
Serpins
Our cells are often forced to work with dangerous machinery. For instance, cells build many machines for demolition, such as nucleases that break down DNA and RNA, amylases and related enzymes that break down carbohydrates, lipases that chew up lipids, and proteases that disassemble proteins. These destructive enzymes are needed in many capacities. They are used in digestion, to break food molecules into workable pieces. They are used in defense, to attack invading viruses and bacteria. They are used to break down defective or obsolete molecules inside cells. They are also used in signaling cascades, to activate signaling molecules instantly when a message is received. These enzymes are essential when used at the proper place and time, but can spell disaster if they get loose.Protection from Proteases
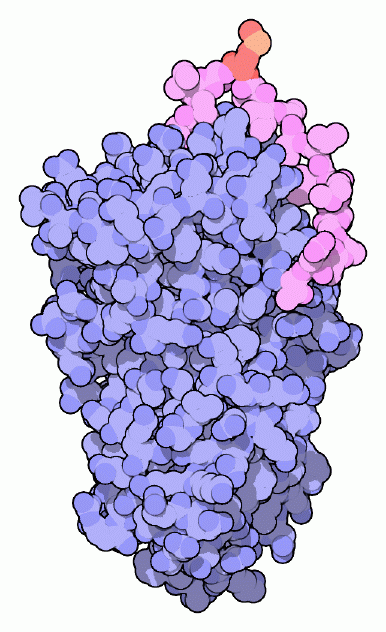
To control these destructive machines, our cells also build a host of proteins that block their action and neutralize the danger. The serpins are one class of these molecules, designed to seek out and destroy specific serine proteases. The name serpin, although sounding like something from Greek mythology, is taken from their function: serine protease inhibitors. The example shown here is alpha1-antitrypsin, from PDB entry 1psi. It is found in the bloodstream, where it protects the surrounding tissues from the protein-cutting enzyme elastase. Neutrophils (a type of white blood cell) secrete elastase in sites of inflammation, where it breaks down connective tissue and allows blood cells to enter and do their jobs in defense and repair. The serpin protects the neighboring areas and ensures that the elastase doesn't spread throughout the body.Trapping Proteases
Serpins are molecular mouse traps, complete with bait and a swinging arm. They are metastable proteins, meaning that they are only partially stable in their active form, but they can snap into a far more stable form when they find a protease. The key is a flexible loop, shown here in magenta. One amino acid in this loop is used as bait. In alpha1-antitrypsin, it is a methionine, shown here in red and orange. As shown on the next two pages, proteases are trapped and destroyed when they take this bait.A Nest of Serpins
Over thirty different human serpins (a number of which are available in the PDB) have been studied, each with a different essential job. Many are found in the blood. Several control the process of blood clotting: antithrombin limits the action of thrombin when a clot is forming, and antiplasmin limits the action of plasmin when blood clots are being disassembled. Other serpins control the action of proteases used in the complement system, which protects us from bacterial infection. For a description of this diverse family of molecules, take a look at the Protein of the Month at the European Bioinformatics Institute.When Serpins Fail
When serpins fail, they can cause serious problems. Two types of problems often occur. A serpin can be defective, so that it is unable to block its proper target and leaves the protease to go on a rampage. This happens in emphysema, where alpha1-antitrypsin is compromised and elastase destroys connective tissue in the lungs. One way that the serpin can be disabled is through smoking, which can modify the methionine amino acid used as bait. Alternatively, the unique trapping mechanism of serpins, shown in more detail on the following pages, can lead to another problem. After the loop is broken, it can associate with other copies of the serpin, leading to bulky aggregates. If these form inside nerve cells, they can block nerve function and lead to dementia.Next: Tripping the Trap
Last changed by: A.Honegger,