|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Aconitase and Iron Regulatory Protein 1
Aconitase is an essential enzyme in the tricarboxylic acid cycle and iron regulatory protein 1 interacts with messenger RNA to control the levels of iron inside cells. You might ask: what do these two proteins have in common? They were discovered and studied by different researchers, who gave them names that described their two very different functions. But surprisingly, when they looked at the amino acid sequence of these proteins, they turned out to be identical. The same protein is performing two very different jobs.
Aconitase...
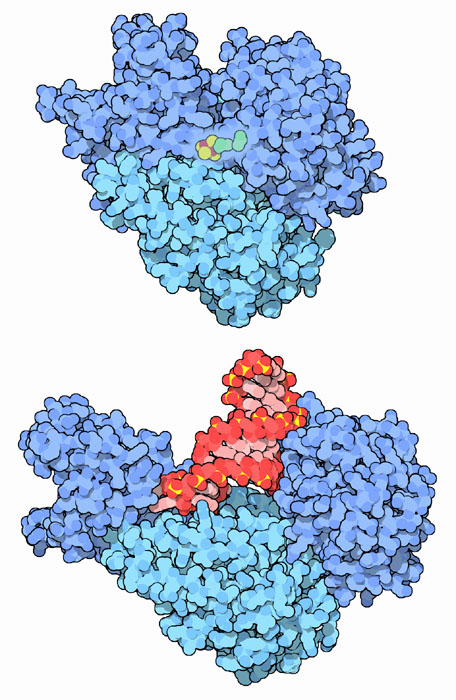
The enzyme aconitase is a key player in the central pathway of energy production. As part of the tricarboxylic acid cycle, it converts citrate into isocitrate. There is one form of aconitase in our mitochondria, which performs most of the conversion for the citric acid cycle, and a similar form in the cytoplasm that creates isocitrate for other synthetic tasks. The cytosolic form is shown here at the top, from PDB entry 2b3y. It is composed of a single protein chain that folds into several domains (the domains are colored slightly different shades of blue here). The domains close like a nutcracker around the active site, which contains an iron-sulfur cluster that assists with the reaction.
...or Iron Regulatory Protein 1
The cytosolic form of aconitase also acts as iron regulatory protein 1. The lower structure, from PDB entry 2ipy, shows how it performs this entirely different function. The iron-sulfur cluster in aconitase is unstable and must be replaced occasionally when it falls out. When iron levels in the cell get low, there isn't enough iron to regenerate the cluster, and the protein shifts to its second function. The protein opens up and grips hairpin loops in a few specific messenger RNA molecules. These include a hairpin at the start of the messenger RNA for ferritin, and five similar hairpins at the end of messenger RNA for the transferrin receptor. When the iron regulatory protein 1 binds, it inhibits the formation of ferritin, so that less iron is locked up in storage, and it enhances construction of the transferrin receptor, so the cell can pick up more transferrin out of the blood, and with it, more iron.
Next: Moonlighting Proteins
Last changed by: A.Honegger,