ClC family
The ClC proteins constitute a large family of transmembrane chloride transport proteins1. The family is widely expressed in all kingdoms of life with an overall conservation of the structural scaffold.
The nine paralogs found in humans show a broad tissue distribution and contribute to diverse processes, such as the control of the resting potential in muscle, transepithelial ion transport in the kidney and acidification of intracellular vesicles. Mutations in family members are associated with severe muscle and kidney diseases such as myotonia, Dents disease and Bartter’s syndrome.
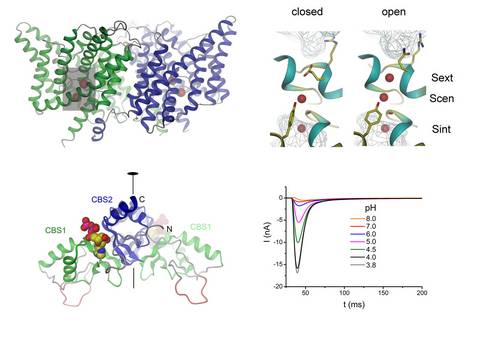
With respect to function, the family shows an unusual diversity in that some of the family members are chloride selective ion channels while other members work as coupled chloride transporters. Based on the first structure determination of a prokaryotic family member working as H+/Cl- antiporter by X-ray crystallography2,3, we have studied the interaction of ions with the protein4 and transport properties in planar lipid bilayers5 and by solid supported membrane electrophysiology6. We also studied the structure of cytoplasmic regulatory domains and their interaction with ligands7-9. The research on this family was supported by grants of the NCCR Structural Biology and the Swiss National Science Foundation.
1 Dutzler, R. The ClC family of chloride channels and transporters. Curr. Opin. Struct. Biol. 16, 439-446 (2006).
2 Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T. & MacKinnon, R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287-294 (2002).
3 Dutzler, R., Campbell, E. B. & MacKinnon, R. Gating the selectivity filter in ClC chloride channels. Science 300, 108-112 (2003).
4 Lobet, S. & Dutzler, R. Ion-binding properties of the ClC chloride selectivity filter. EMBO J. 25, 24-33 (2006).
5 Accardi, A., Lobet, S., Williams, C., Miller, C. & Dutzler, R. Synergism between halide binding and proton transport in a CLC-type exchanger. J. Mol. Biol. 362, 691-699 (2006).
6 Garcia-Celma, J., Szydelko, A. & Dutzler, R. Functional characterization of a ClC transporter by solid-supported membrane electrophysiology. J. Gen. Physiol. 141, 479-491 (2013).
7 Meyer, S. & Dutzler, R. Crystal structure of the cytoplasmic domain of the chloride channel ClC-0. Structure 14, 299-307 (2006).
8 Markovic, S. & Dutzler, R. The structure of the cytoplasmic domain of the chloride channel ClC-Ka reveals a conserved interaction interface. Structure 15, 715-725 (2007).
9 Meyer, S., Savaresi, S., Forster, I. C. & Dutzler, R. Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5. Nat. Struct. Mol. Biol. 14, 60-67 (2007).