Navigation auf uzh.ch
Navigation auf uzh.ch
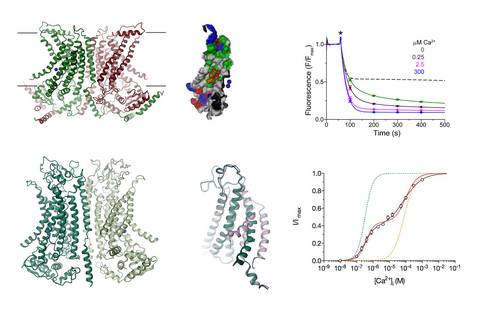
The TMEM16 proteins constitute a family whose members either function as calcium-activated anion channels or lipid scramblases17. The family is only present in eukaryotes and contains 10 paralogs in humans, which are broadly distributed and participate in important physiological processes. Mutations in TMEM16 proteins lead to bleeding disorders and ataxia. The anion channels TMEM16A and B contribute to epithelial chloride transport and the control of electrical signaling in smooth muscles and certain neurons. The presence of TMEM16A in airway epithelia makes this protein a promising drug target target against cystic fibrosis. Family members working as lipid scramblases are either found on the plasma membrane or intracellular organelles. In platelets, the scramblase TMEM16F participates in the activation of the blood coagulation cascade by mediating the exposure of the lipid phosphatidylserine to the outside of cells.
By determining the X-ray structure of the close fungal homologue nhTMEM16, which functions as lipid scramblase, our work has revealed the general architecture of the family18. This structure has also provided first insight into the properties of lipid scramblases, which provide a polar pathway for lipid head-groups on their way across the membrane. We subsequently characterized the functional differences distinguishing lipid scramblases from ion channels within the TMEM16 family. We have used electrophysiology to demonstrate the functional independence of the two ion-conduction pores in the dimeric protein TMEM16A19 and the role of positively charged residues for anion conduction20. By using cryo-electron microscopy, we have later determined structures of the ion channel TMEM16A in ligand-bound and free state, which has revealed its structural changes upon activation21.
Current work focuses on the detailed functional mechanisms of channels and scramblases and the characterization of the lipid scramblase TMEM16F. The research on this family is supported by an advanced investigator grant of the European Research Council (ERC).
17 Brunner, J. D., Schenck, S. & Dutzler, R. Structural basis for phospholipid scrambling in the TMEM16 family. Curr. Opin. Struct. Biol. 39, 61-70 (2016).
18 Brunner, J. D., Lim, N. K., Schenck, S., Duerst, A. & Dutzler, R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516, 207-212 (2014).
19 Lim, N. K., Lam, A. K. & Dutzler, R. Independent activation of ion conduction pores in the double-barreled calcium-activated chloride channel TMEM16A. J. Gen. Physiol. 148, 375-392 (2016).
20 Paulino, C. et al. Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. eLife 6, doi:10.7554/eLife.26232 (2017).
21 Paulino, C., Kalienkova, V., Lam, A. K. M., Neldner, Y. & Dutzler, R. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature 552, 421-425 (2017).